Project News & Updates
Why are we doing this study?
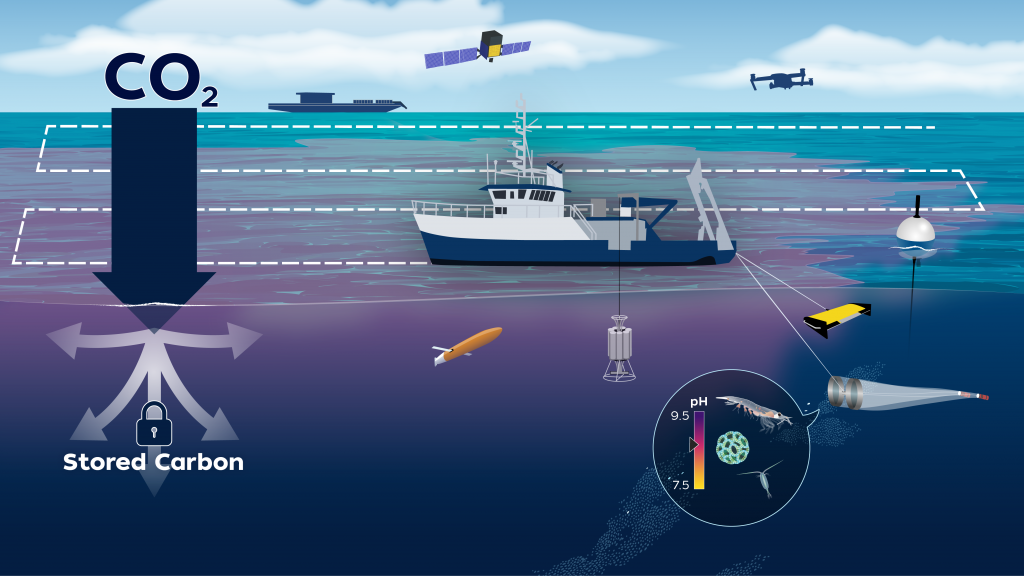
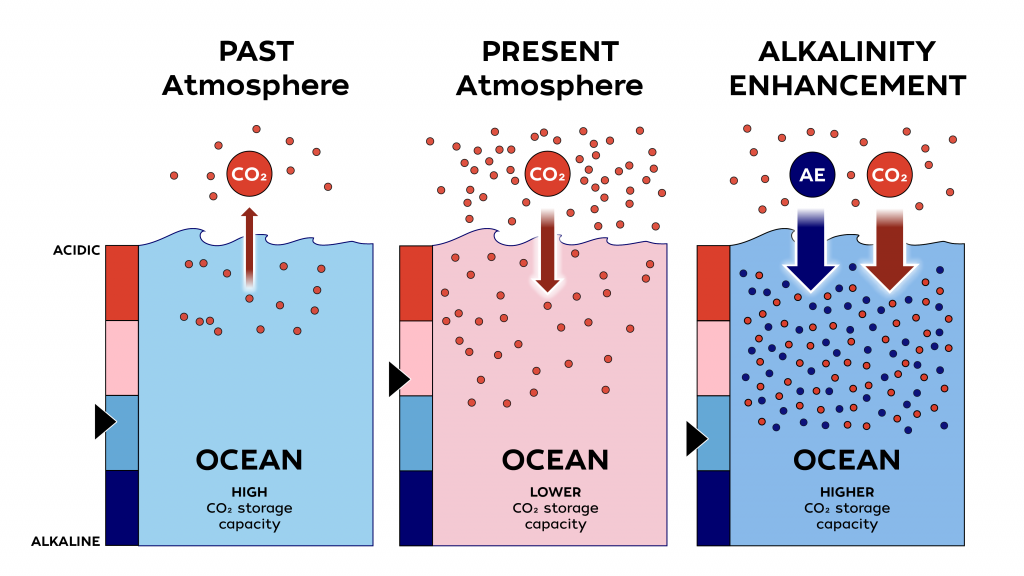
Can ocean alkalinity enhancement help remove carbon dioxide from the atmosphere?
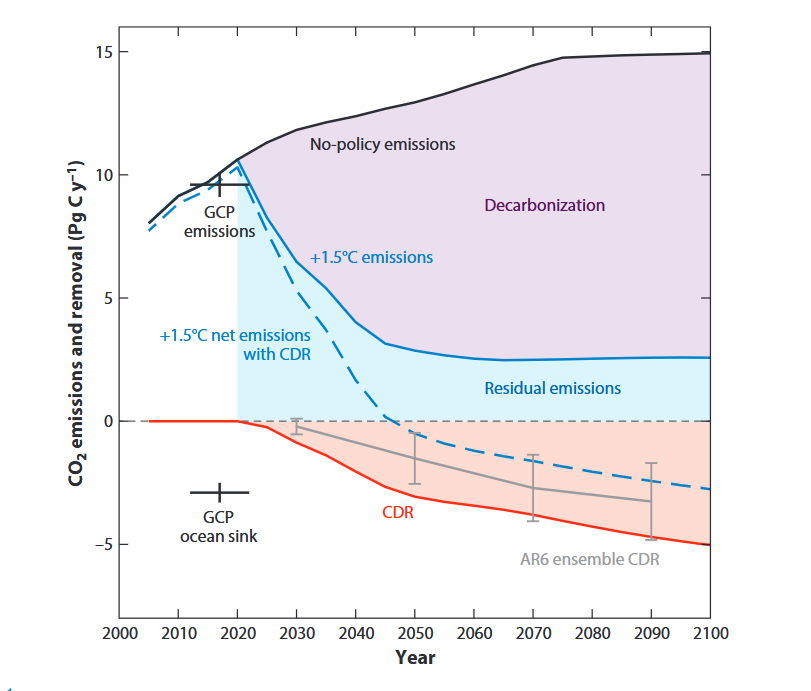
The LOC-NESS project (short for Locking Ocean Carbon in the Northeast Shelf and Slope) is a research effort that addresses the need identified by the US Federal Government and the UN’s Intergovernmental Panel on Climate Change to rapidly advance research into marine Carbon Dioxide Removal (mCDR) approaches, such as ocean alkalinity enhancement (OAE), a potential type of mCDR that de-acidifies sea water while storing carbon away from the atmosphere. As a supplement to emissions reductions, OAE may help to reduce the impacts of climate change on the environment and society. Through a voluntary carbon market, startup companies are already selling carbon credits via OAE. Highly monitored, carefully designed research projects such as LOC-NESS are critical to providing scientific assessments of the potential risks and benefits of these new and rapidly developing climate solutions.
Who are we?
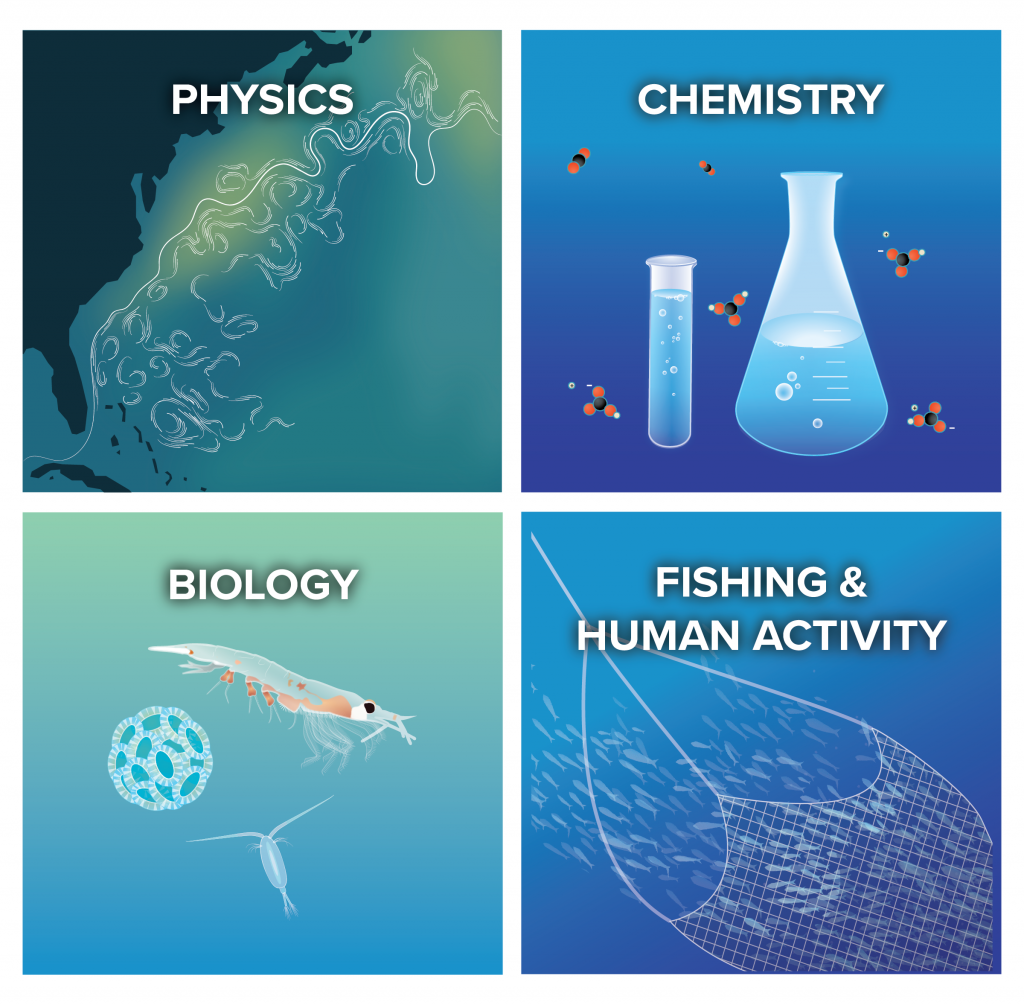
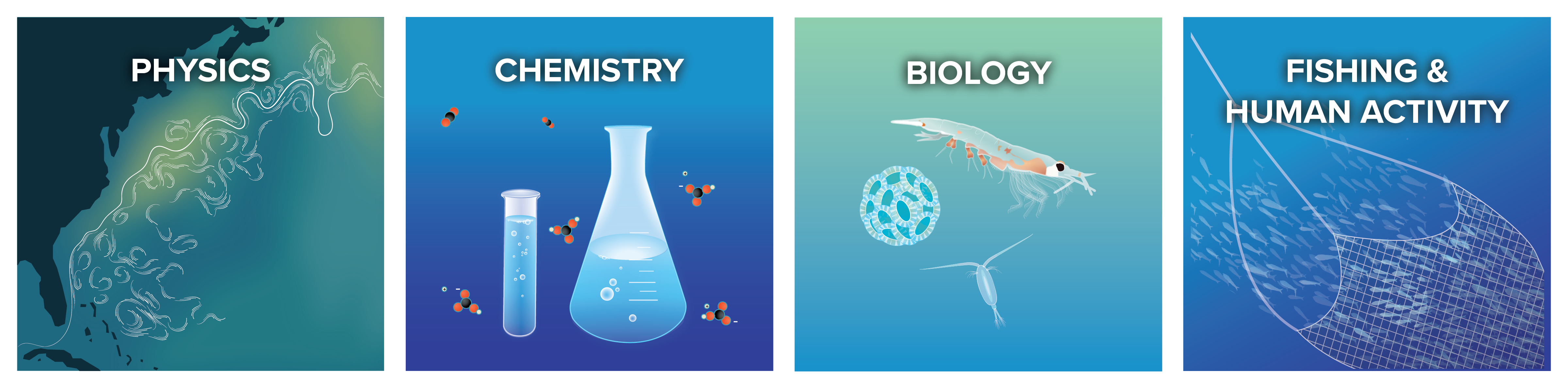
We are an interdisciplinary team of scientists, engineers, and communicators who are committed to a transparent, rigorous, scientific evaluation of ocean alkalinity enhancement.
This research project is not a pathway to, or an endorsement for, pursuing OAE as a large-scale carbon dioxide removal strategy.